We provide advice to NHS boards and their Area Drug and Therapeutics Committees (ADTCs) across Scotland about the status of all newly licensed medicines and all new formulations and new indications of established products.
We aim to make this advice available as soon as practical after the launch of the product involved.
The SMC remit is confined to prescription only medicines (POMs). We do not assess:
- vaccines
- generics
- pharmacy and general sales list medicines
- blood products, and
- diagnostics.
Devices that contain a medicine are only assessed if they have been licensed as a medicine by the Medicines and Healthcare products Regulatory Agency (MHRA).
Details of how we work with pharmaceutical companies can be found in our guide, Working with SMC - a guide for manufacturers. Our full submission process requires companies to complete a New Product Assessment Form (NPAF).
The Association of the British Pharmaceutical Industry (ABPI), in partnership with SMC, has developed a toolkit for industry representatives to help them with their submissions. For more advice on making a submission, please get in touch via our contact us page.
In the event that the SMC secretariat has not communicated directly with a company to discuss submission plans, companies are required to communicate with us via our contact page prior to making a submission or resubmission.
All documents relating to company submissions can be viewed and downloaded from this page.
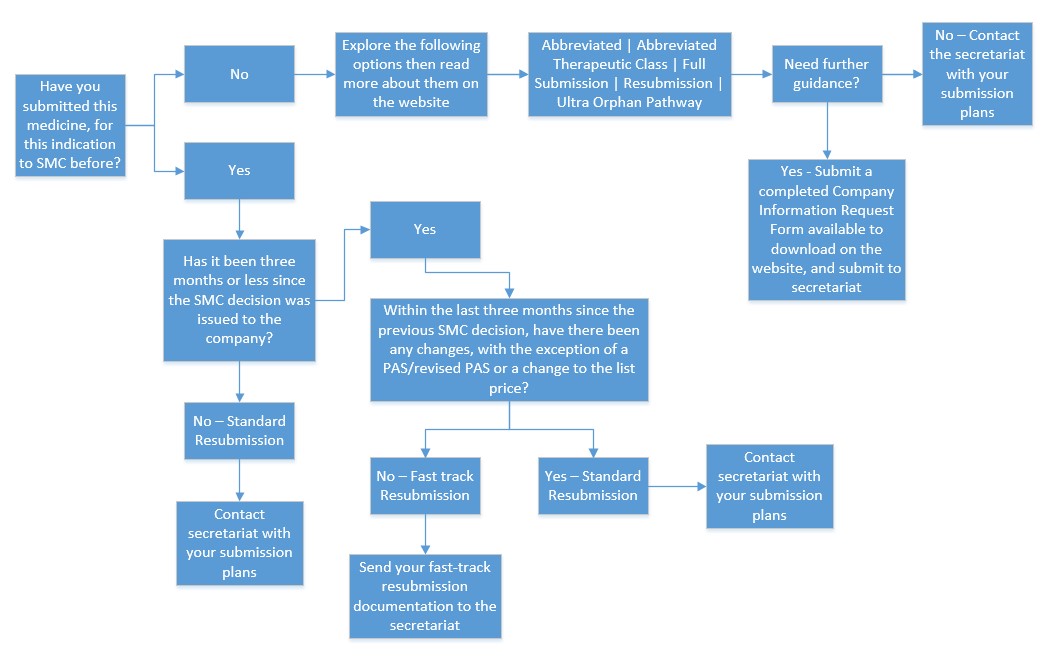
Choosing a relevant submission type
Understanding how new medicines impact the quality of life of patients and carers is an important part of the SMC decision making process.
We work in partnership with patient groups from around the country to capture the experiences of patients, their families and carers via our patient group submission process. Our Guide for patient group partners explains what type of information you need to capture the views and experiences of the patients and carers you represent.
-
Guide For Patient Group Partners 2022 1mb (PDF)
Download guide
Support to make your submission
The Public Involvement team are able to guide you through the submission process and are available by email or to speak to on the phone. If it would be of help to you, they can also meet with you to support you.
To provide a patient group submission for a medicine you complete a patient group submission form. If you have any problem accessing the electronic version of the form we can either email it to you or send you a paper version in the post. Please remember you will need to fill in a patient group registration form before you make a submission.
You will usually have between six and eight weeks from when the appraisal is announced to complete and return your submission. The Public Involvement team will advise you on exact timelines for each submission.
The information you collect and submit will be used by the assessment team, along with other information, to prepare its reports and by the appraisal committee to develop its recommendations.
All documents relating to patient group submissions can be viewed and downloaded from this page.
You can find out more about patient group submissions by watching our short film detailing a patient group's experience of the process.
Preparing a submission for SMC - the patient group experience
Watch one of our patient group partners describe their experience of making a submission.
-
Patient group submission example (ADHD) 261kb (PDF)
Download
A company may make a submission for a product once it has received a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) or up to two months before final approval from the Medicine and Healthcare products Regulatory Agency (MHRA).
In the event that the SMC secretariat has not communicated directly with a company to discuss submission plans, companies are required to communicate with us via our contact page prior to making a submission or resubmission.
The usual assessment timeline is 18 weeks, i.e. from scheduling of a submission to publication of advice. A longer timeline, e.g. 22 - 26 weeks, is required for submissions for:
- end of life / orphan medicines that require a Patient and Clinician Engagement (PACE) meeting
- medicines with a complex patient access scheme (PAS), and
- occasionally for complex submissions e.g. ones which include multiple clinical studies or analyses.
In periods where a high number of submissions are received we may need to prioritise submissions to meet the needs of patients, prescribers and the healthcare system.
When it is necessary to prioritise submissions for assessment we take into account factors including:
- Innovative Licensing and Access Pathway (ILAP): Lead indication for Innovation Passport designation
- Patient need: As agreed with NHS boards and our industry User Group Forum, we may prioritise submissions for medicines where there is exceptionally high patient need. For example, a submission for a medicine used for a condition where no other treatment is available may be prioritised over one where an alternative treatment is available
- Health service need: This includes consideration of, for example, a situation where an unlicensed product is currently being used or where an existing treatment requires a specialist service for delivery
- Date submission received
- Date that product is due to become available in the UK: A submission for a medicine that is already available may be prioritised over others that do not yet have marketing authorisation, and
- Patient Access Scheme Assessment Group (PASAG) timeline: If a submission involves a complex PAS, NDC will not consider the medicine until a PASAG decision on the operational feasibility of the scheme is available.
-
Company Submission Dates 154kb (DOCX)
Download

