We provide advice to NHS boards and their Area Drug and Therapeutics Committees (ADTCs) across Scotland about the status of all newly licensed medicines and all new formulations and new indications of established products.
We aim to make this advice available as soon as practical after the launch of the product involved.
The SMC remit is confined to prescription only medicines (POMs). We do not assess:
- vaccines
- generics
- pharmacy and general sales list medicines
- blood products, and
- diagnostics.
Devices that contain a medicine are only assessed if they have been licensed as a medicine by the Medicines and Healthcare products Regulatory Agency (MHRA).
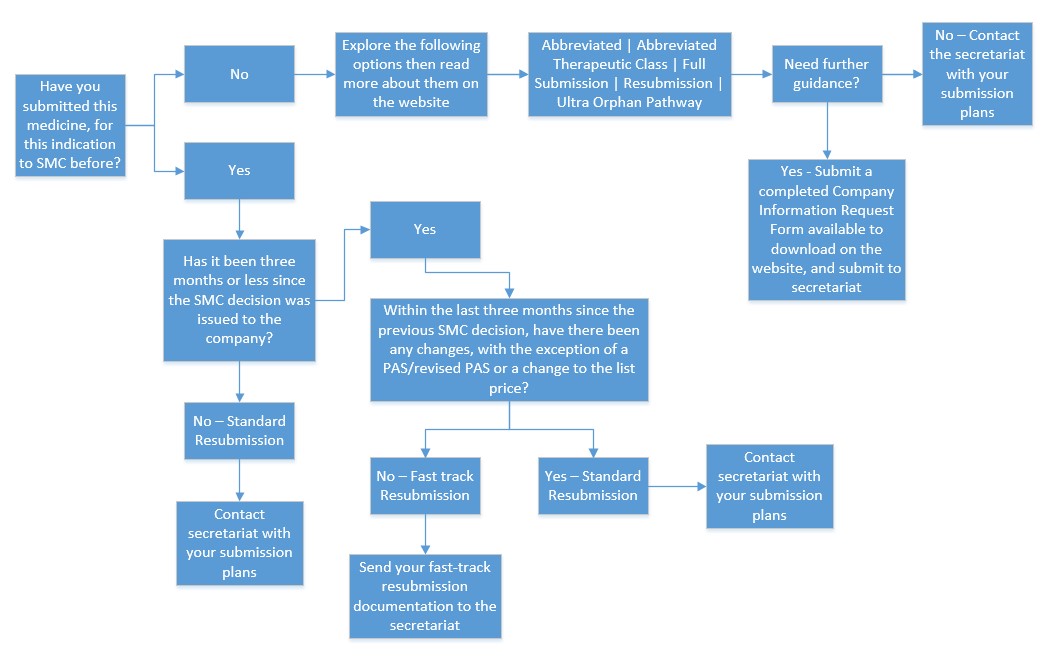
Details of how we work with pharmaceutical companies can be found in our guide, Working with SMC - a guide for manufacturers. Our full submission process requires companies to complete a New Product Assessment Form (NPAF).
The Association of the British Pharmaceutical Industry (ABPI), in partnership with SMC, has developed a toolkit for industry representatives to help them with their submissions. For more advice on making a submission, please get in touch via our contact us page.
In the event that the SMC secretariat has not communicated directly with a company to discuss submission plans, companies are required to communicate with us via our contact page prior to making a submission or resubmission.
All documents relating to company submissions can be viewed and downloaded from this page.
Choosing a relevant submission type